Below please find answers to commonly asked questions. If you do not find the answer to your question, please contact us at: info@mediclever.com
Reimbursement Questions
- How long does it take to obtain reimbursement?
- How much does it cost?
- Where should we start? In which country should we launch our first reimbursement project?
- When should we start looking into reimbursement issues?
- Our product is already reimbursed in the USA. Will it help us obtain reimbursement in Europe?
- Is reimbursement in Europe similar for all EU countries?
- We have an excellent distributor that we count on. Should we let him/her handle our reimbursement needs?
Reimbursement answers
The first thing you should do is check whether your device fits within an existing reimbursement mechanism (code, coverage and payment rate) or in case of a drug, whether it fits within a group of comparable pharmaceutical products, for which maximum reimbursement prices have already been set. This is typically conducted as part of our Step 1 activity (Reimbursement Landscape) report.
In case your product fits within an existing mechanism/group, reimbursement is almost immediate.
In case there is no existing reimbursement mechanism/group, developing a new reimbursement mechanism could be a matter of years, depending on the product, its benefits the political and economical environment and many other factors. However, even in such cases, before all reimbursement mechanisms have been established, there are usually short-term, intermediate reimbursement mechanisms that could provide your drug / device with some sort of reimbursement within months. Please refer to our Resources page for a few examples.
To view the above explanation graphically, check out our Synchronizing Reimbursement and Regulatory Activities Presentation.
Naturally, the cost of each reimbursement project may vary according to the product, the country and the relevant reimbursement-related environment.
To receive a business proposal that matches your company's needs, please click here and a Mediclever representative will contact you shortly.
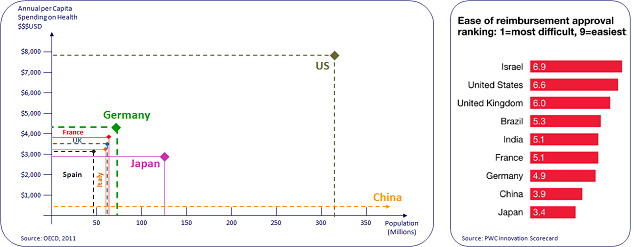
Most of our clients typically start with the USA, Germany and the UK as their first markets, followed by France, Italy and Spain as their second tier.
Some of the main considered criteria are market size and the ease of reimbursement process in each market.
As can be seen in the following graphs, the combination of market size and ease of reimbursement process supports our clients' decision.
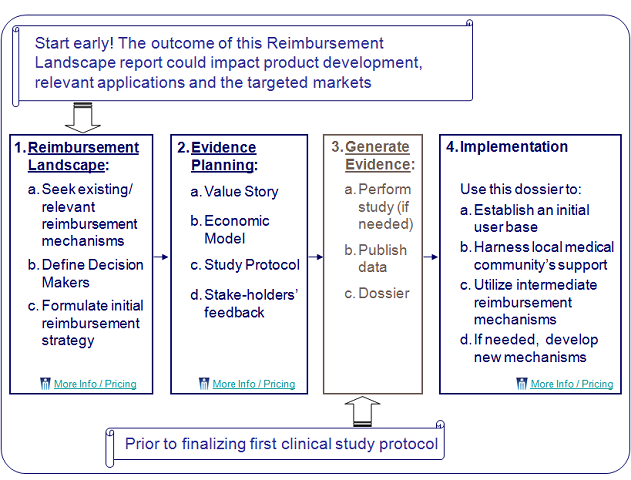
- Reimbursement Landscape: Should be conducted as early as possible as it could impact product development, relevant applications and the targeted markets.
- Evidence Planning: Should be completed prior to initiating phase III studies as this step may impact the design of a clinical study.
- Implementation: A few months prior to launching the new product.
To learn more, please refer to our Services page.
Typically, there is no formal link between reimbursement systems in these countries. However, decision makers become increasingly receptive to reimbursement related data from other countries that may affect their decision making process.
No. As opposed to the general CE mark, each country maintains its different reimbursement mechanisms and reimbursement in one European country does not imply reimbursement in another.
- We have an excellent distributor that we count on. Should we let him/her handle our reimbursement needs?
A distributor with reimbursement experience may assist in checking existing reimbursement mechanisms, but should not determine the company's reimbursement strategy or apply for any sort of reimbursement by himself/herself.
For more on this subject, please refer to the article: "Reimbursement by Distributors".

